Across Europe, groundbreaking ideas emerge from SMEs, universities, and research consortia. Public programs like Horizon Europe, ITEA, and EuroStars provide vital early funding, creating fertile ground for breakthroughs. Yet translating EU-funded research outputs into real-world impact (commercial, scientific, societal, or policy-driven) is rarely straightforward, and many promising projects stall before reaching their full potential.
For research teams, adoption is the gateway to recognition and growth; for funders, investors, and support organizations, helping innovations scale generates returns and societal value. Overcoming adoption challenges not only unlocks technical results but also accelerates market entry, increases impact, and maximizes return on investment. The central question is: How can we accelerate adoption and unlock the full value of promising work? This post outlines practical strategies to bridge the gap, with a focus on commercialization while remaining relevant to research, societal, and policy outcomes.
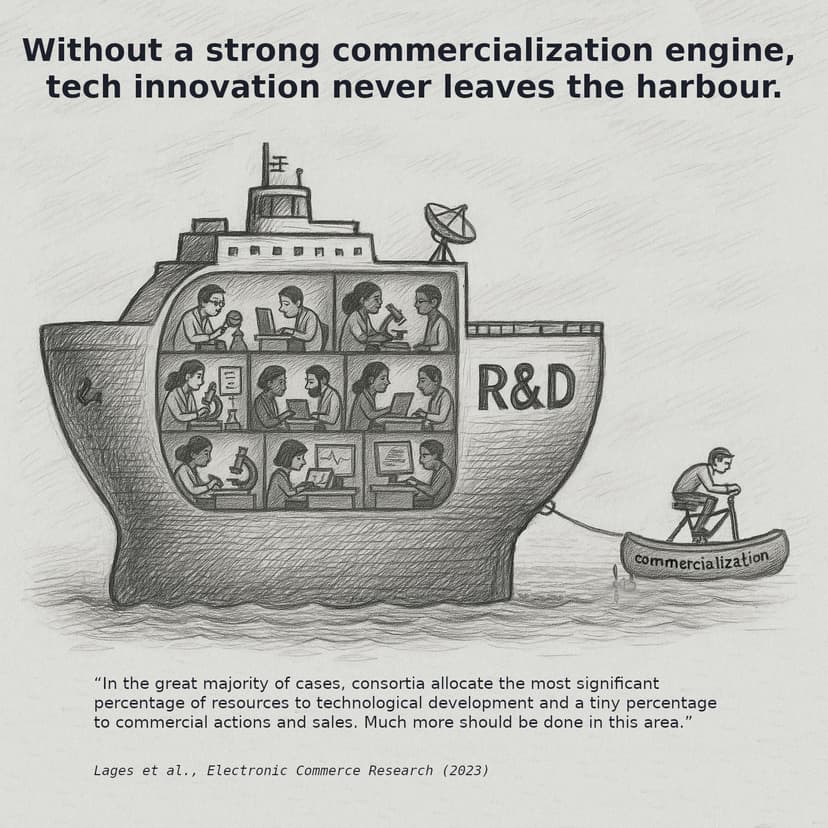
Lessons from EU-Funded R&D Projects
Having worked on numerous EU- and Dutch-funded R&D projects, I’ve seen firsthand how even promising innovations can stall before reaching adoption. A recurring challenge is that concrete commercialization activities are often under-resourced, even when funding bodies emphasize their importance.
"Surprisingly, even in the case of EC Innovation Actions, where large consortia are expected to have commercial success, in the great majority of cases, consortia allocate the most significant percentage of resources to technological development and a tiny percentage of their total budget to commercial actions and sales. Much more should be done in this area."
- Solutions for the commercialization challenges of Horizon Europe and earth observation consortia by Lages, L.F. et al. (Electronic Commerce Research)
While this quote focuses on earth observation and ICT projects, its core lesson resonates across sectors: proactive attention to innovation adoption is essential to maximizing value. Funding programs prioritize technological development. However, their structure can limit resources and flexibility for commercialization and adoption. Recognizing these structural limitations early allows teams to embed adoption-focused planning from the start, increasing the likelihood that innovations scale successfully.
This post provides a practical, step-by-step approach to systematically test assumptions, validate market relevance, and steer research toward adoption-ready outcomes—so that your innovation doesn’t stall in the harbor.
Example Scenario: Bringing a New Medical Device to Market
To illustrate how research teams can apply each step in practice, this blog explores a hypothetical innovation project.
- Project: Wearable patch + cloud algorithm to flag early atrial fibrillation (AFib)
- Aim: Earlier detection means fewer hospitalizations and a better quality of life
- Constraint: Must prove accuracy and comply with EU and international regulations while fitting existing clinical workflows
1. Clarify the Research Goal and Impact Pathways in Innovation Projects
Before a project can move toward adoption, it’s important to establish a clear understanding of what the research aims to achieve and why it matters. This step sets the foundation for aligning the team, engaging stakeholders, and ensuring that innovation efforts deliver measurable outcomes. The following key actions help translate this understanding into an adoption-ready plan:
- Define the problem clearly: Articulate the core issue the research aims to solve and why it matters.
- Set intended impact metrics: Describe how success will be measured in clinical, societal, or market terms.
- Identify key stakeholders early: Map out who benefits, who decides on adoption, and who integrates the solution.
- Align team vision around adoption: Ensure all contributors understand that success goes beyond publications and project deliverables.
Example — Step 1: Clarify Goal & Impact
In the AFib wearable project, the team anchors their work around a clear medical and adoption-oriented objective. By defining measurable impact, they can align research activities with both clinical and market needs.
- Problem statement: “Undiagnosed AFib leads to avoidable strokes and hospital admissions.”
- Intended impact: Reduce detection-to-treatment time by 30% within 12 months of deployment.
- Stakeholder focus: Cardiologists (clinical confidence), patients (comfort/adhesion), hospital IT (integration effort), payers (cost offsets).
2. Map Adoption and Commercialization Assumptions Early
Before diving into experiments or development, it’s essential to identify the assumptions underlying your project. Understanding what you believe about desirability, feasibility, and viability—and which assumptions are most critical—helps focus effort where it matters most. Early assumption mapping creates a clear roadmap for testing and validation, reducing wasted resources and uncovering hidden risks.
- Take an inventory of assumptions: Document hypotheses about who will benefit (desirability), whether it can be done (feasibility), and the resources or scalability required (viability).
- Prioritize the most critical: Identify which assumptions, if wrong, would block adoption and focus on testing these first.
- Turn assumptions into questions: Frame each assumption as a concrete question to guide experiments, evidence gathering, and decision-making.
Example — Step 2: Map Early Assumptions
Mapping assumptions surfaces where the project could stall if left untested. For the AFib wearable, this means identifying what must be true for clinicians, patients, hospitals and those that pay for the intervention. Here are a few assumptions that the research team might need to test:
- Desirability: Patients will wear a chest patch 8+ hrs/day for 14 days; Clinicians trust alerts.
- Feasibility: Sensitivity/specificity meets clinic thresholds vs. 12-lead ECG benchmark.
- Viability: Hospitals are willing to adopt the innovation if it reduces readmissions.
- Affordability: Someone is willing to pay for the intervention (hospital and/or health insurers).
3. Design Early Experiments to Validate Research Adoption and Market Fit
Validating both technical feasibility and market relevance early in a project is critical to prevent wasted effort and stalled innovations. By testing assumptions through tangible experiments and engaging potential users or stakeholders, teams can gain actionable insights that guide research toward real-world adoption.
- Create a simple business plan: Fill in the one-page Lean Canvas or the Leaner Lean Canvas.
- Engage stakeholders early: Conduct interviews, co-design workshops, or pilot tests to validate desirability and identify practical constraints.
- Develop prototypes or demonstrators: Build small-scale proof-of-concepts to test whether the solution works as intended.
- Implement iterative learning loops: Continuously test, capture feedback, refine experiments, and repeat to adapt quickly and reduce risk.
Example — Step 3: From Leaner Lean Canvas to Early Experiments
The AFib wearable team starts with a Leaner Lean Canvas to capture adoption-critical pieces:
Customer Segments: Cardiology departments in EU hospitals; patients at elevated AFib risk
Early Adopters: Digital-forward cardiology units open to remote monitoring programs
Problems: Missed paroxysmal AFib; clinician overload from noisy data; poor patient adherence
Existing Alternatives: 24–48h Holter; intermittent ECG; smartwatch notifications; clinic follow-ups
With this as their starting point, the team quickly moves into early experiments:
- Engaging stakeholders through interviews with cardiologists to check whether the proposed alerts are seen as clinically useful.
- Building a mini demonstrator with a small dataset to show that their cloud algorithm can reliably distinguish AFib episodes from noise.
- Running iterative loops: each round of testing surfaces new insights, which they capture by refining their Leaner Lean Canvas. Over time, this evolves into a full Lean Canvas as constraints are removed and assumptions validated.
By linking lightweight planning, stakeholder input, and rapid prototyping, the team avoids wasted effort and uncovers adoption barriers early.
Learn more about applying agile and lean principles to accelerate EU-funded research in Turning Slow Grants into Fast Innovation: Agile Strategies for EU Funding and Lean Thinking for Innovation Impact.
4. Align Research Outputs with Market and Policy Context
Even the most innovative research can fail to create impact if outputs are misaligned with the realities of adoption. To increase the chances of real-world uptake, teams should evaluate regulatory, policy, data, and economic factors early. Considering these aspects from the start helps prevent downstream obstacles and accelerates the path from innovation to implementation.
- Regulatory compliance: Identify classification and ensure alignment with ISO standards, and other sector-specific requirements.
- Policy alignment: Anticipate upcoming EU regulations (e.g., AI Act) that may shape trust, transparency, and deployment.
- Data & integration: Plan GDPR-compliant data handling and ensure smooth interoperability with existing systems and workflows.
- Economic feasibility: Model costs, scalability, and reimbursement pathways to support long-term sustainability.
Example — Step 4: Align with Adoption & Regulatory Context
Adoption in medical devices hinges not just on performance, but also on compliance and integration. For the AFib wearable, the team considers the Medical Device Regulation (MDR), the AI Act, and operational realities early.
- MDR & ISO 13485: Define intended use, likely Class IIa; begin clinical evaluation plan, risk management (ISO 14971), and software lifecycle documentation (IEC 62304).
- AI Act: Since the wearable uses an algorithm to detect AFib, the team prepares for transparency, explainability, and post-market monitoring requirements under the Act.
- Data & integration: Design GDPR-compliant workflows and test interoperability with EHR systems (HL7/FHIR if applicable).
- Economics: Build a model for avoided hospital admissions and reduced clinician time, then explore potential reimbursement pathways.
5. Create a Roadmap Linking R&D Outputs to Innovation Adoption Goals
A development roadmap provides a clear path from research outputs to real-world adoption. It helps teams prioritize experiments, coordinate technical and adoption milestones, and reduce uncertainty. By planning both scientific validation and adoption-related activities, teams can accelerate progress and ensure the innovation reaches end users effectively.
- Define dual-track milestones: Map TRL progression alongside adoption targets such as pilot tests, integration into target environments, and stakeholder training.
- Prioritize critical experiments and resources: Allocate budget, personnel, and infrastructure to the highest-impact technical and adoption-related activities.
- Plan regular review cycles: Schedule check-ins with stakeholders and regulatory or domain experts to monitor progress and de-risk the pathway to adoption.
Example — Step 5: Roadmap to Adoption
A roadmap connects technical validation with adoption milestones. The AFib project plans its trajectory so progress toward CE-marking and pilot use happens in parallel.
- Milestones: TRL5 functional prototype; TRL7 clinical pilot; TRL8/9 CE-mark & first deployments.
- Dual tracks: Pair technical milestones (accuracy, robustness) with adoption milestones (completed pilot studies, integration with clinical workflows, and clinician training).
- Schedule: Hold quarterly adoption reviews with cardiology leads and periodic regulatory check-ins to de-risk MDR submissions.
6. Foster a Research Culture Focused on Adoption and Real-World Value
Creating a culture that prioritizes adoption and real-world impact is just as important as technical achievement. Teams that integrate impact-oriented thinking, embrace iterative learning, and engage external perspectives are better positioned to move innovations toward meaningful outcomes.
- Impact-oriented thinking: Align team discussions around user needs, societal or market impact, and overall project objectives to ensure everyone understands the broader goals.
- Iterative learning: Treat experiments and early prototypes as opportunities to learn. Document results, including failures, and adjust assumptions or processes accordingly.
- External engagement: Seek feedback from relevant stakeholders, domain experts, or end users early to validate decisions and uncover hidden adoption risks.
Example — Step 6: Outcome-Oriented Culture
By embedding these principles into daily practice, the AFib team keeps adoption in sight while tackling scientific challenges:
- Rituals / Impact-oriented thinking: Monthly "impact check-ins" covering patient value, clinician workload, and safety signals.
- Learning loop / Iterative learning: Log experiment outcomes, including failures, and adjust assumptions and project direction.
- External voices / External engagement: Engage a regulation-savvy advisor, a reimbursement expert, and a patient advocate early to guide decisions and validate adoption pathways.
For a deeper dive into the human factors that can hinder disruptive innovation and how to overcome them, see Innovation Lives Outside of the Comfort Zone.
Conclusion
Effective adoption of R&D outputs requires more than strong technical results—it demands deliberate planning, validation, and alignment with real-world needs. By clarifying goals, systematically testing assumptions, and ensuring outputs are adoption-ready, teams can prepare promising research for tangible impact.
To maximize outcomes, consider implementing structured experiments, engaging stakeholders early, and fostering a culture oriented toward adoption. For a deeper understanding of why promising research sometimes fails to reach impact, see Why Good Research Gets Overlooked: 6 Hidden Barriers in the Impact Pipeline.
Drawing on experience with EU- and Dutch-funded projects, I work with teams through Eunovus to provide agile project management and software development support that helps research move from innovation to market impact.
Move innovation to adoption faster. Eunovus provides agile project management and software development support to ensure your research reaches its full potential. Let’s discuss your next project.
